|
|
|
Dear {{customText[Dr.|Mr.|Mrs.|Ms.| |]}} {{accLname}},
|
|
|
|
|
AN OPTION AVAILABLE FOR IN-OFFICE ADMINISTRATION
|
| |
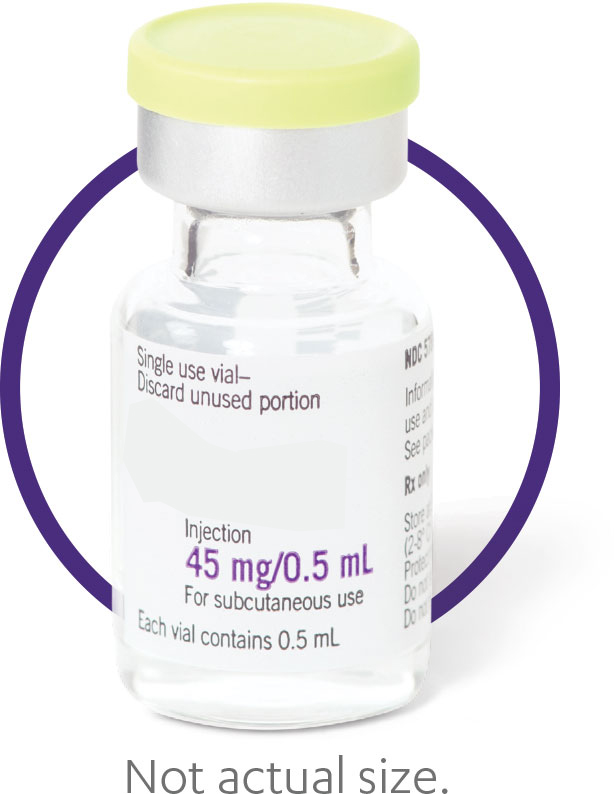
| Single-dose 45‑mg vial—available as a medical benefit option for subcutaneous injection |
| |
|
| |
Patients need only 4 maintenance injections per year (after 2 starter injections at Week 0 and Week 4)1 |
| |
May be right for your patients with active PsA who may benefit from in-office administration |
| |
Single-dose 45-mg solution in vial requires no reconstitution1 |
| |
Streamlines the reimbursement process with a permanent J-Code*: J33573 |
| * |
Codes are supplied for informational purposes only and represent no statement, promise, or guarantee that reimbursement will be made. Information provided is not intended to increase or maximize reimbursement. |
|
|
| |
| BRAND® DOSING IN ACTIVE PsA1 |
| • |
The recommended dose is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks |
|
| • |
For patients with co-existent moderate-to-severe plaque psoriasis weighing >100 kg (220 lbs), the recommended dose is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks |
|
|
| BRAND®, available as 45 mg and 90 mg prefilled syringe and 45 mg single dose vial, is a subcutaneous injection intended for use under the guidance and supervision of a physician with patients who will be closely monitored and have regular follow-up visits with a physician. If a physician determines that it is appropriate, a patient may self-inject or a caregiver may inject BRAND® after proper training in subcutaneous injection technique. Patients should be instructed to follow the directions provided in the Medication Guide.1 |
| |
|
References:
1. BRAND® [Prescribing Information]. XXXX, XX: Company Tech, Inc; XXXX. 2. Data on file. Company Tech., Inc.
|
| |
| If you no longer wish to receive emails from Company Tech., Inc., please unsubscribe. | Privacy Policy |
| |
|
Company Tech., Inc. | XXX XXXXXXX XX | XXXXXXX, XX XXXX
|
| © Company Tech., Inc. XXXX XX/XX XX-XXXXXXX |
| |
|
|